Result
Construction of Plasmid Carrying the GFP genes
Test whether the extracted plasmids from transformed E.coli are correct
Agarose gel electrophoresis of digested plasmids
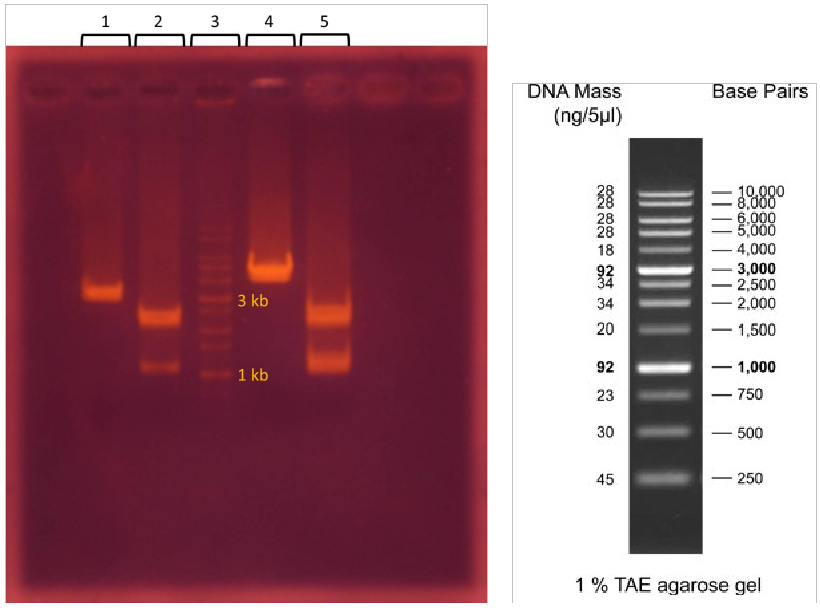
2. T7-promoter GFP plasmid + EcoRI & PstI
3. 1 kb DNA ladder
4. lacI regulated GFP generator plasmid + EcoRI
5. lacI regulated GFP generator plasmid + EcoRI & PstI
* T7-promoter GFP plasmid stands for pSB1C3 - BBa_I746909 plasmid
* lacI regulated GFP generator plasmid stands for pSB1C3 - BBa_K082034 plasmid
T7-promoter GFP plasmid is 2796 bp and lacI regulated GFP generator plasmid is 3189 bp.
After EcoRI digestion and electrophoresis analysis, the molecular size of T7-promoter GFP plasmid is about 2800 bp and lacI regulated GFP generator plasmid is about 4000 bp.
After EcoRI & PstI digestion and electrophoresis analysis, T7-promoter GFP plasmid is separated into two sequences, 1000 and 2000 bp, and lacI regulated GFP generator plasmid is also separated into two sequences, 1100 and 2000 bp.
The result of EcoRI digestion reveals that the molecular size of lacI regulated GFP generator plasmid is larger than we expect, thus we decide to conduct electrophoresis of digested lacI regulated GFP generator plasmid again.
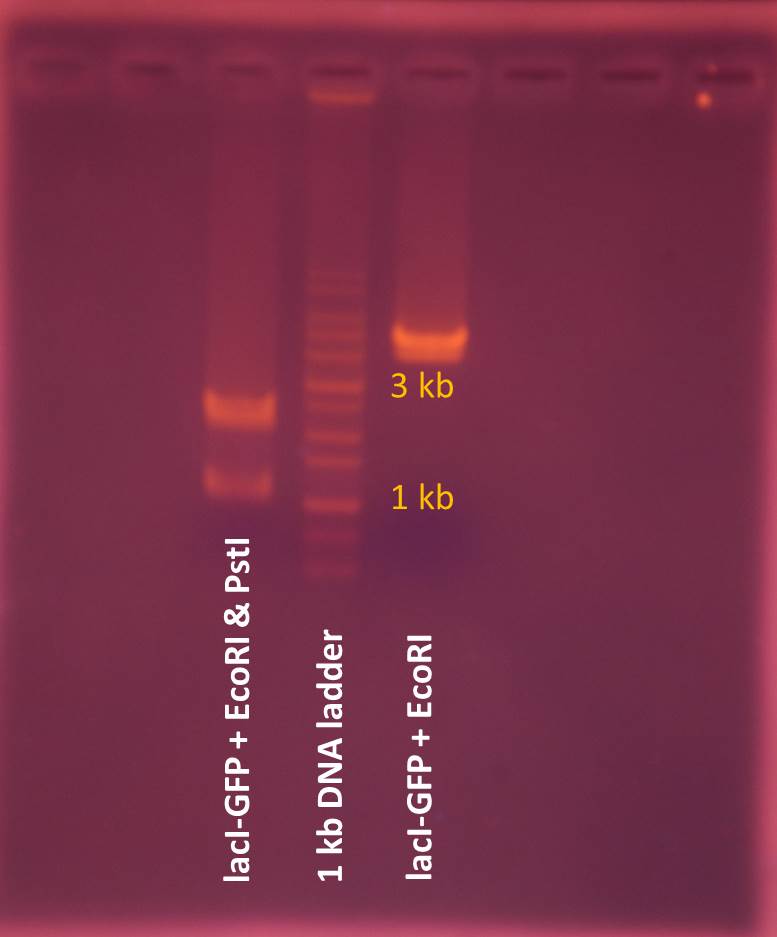
The result of electrophoresis shows that lacI regulated GFP generator plasmid is still about 4000 bp and doesn’t correspond to the information provided by iGEM, thus we choose T7-promoter-GFP plasmid to be delivered by NanoNeedle.
Find The Best Concentration of Magnesium Ion in The Origami Folding Process -Agarose Gel Electrophoresis

*Control: the non-folding mixture of scaffolds and staples at 8 mM MgCl2. We use the control to compare the effect of folding reaction.
Since the concentration of magnesium ion affects the structure of DNA origami significantly, in the beginning of experiment we try 8 different concentrations from 8 mM to 22 mM MgCl2 in folding reaction.
The electrophoresis result reveals that after the folding reaction, bands of scaffolds in the mixture of scaffolds and staples at 14, 16 and 18 mM MgCl2 are less obvious than only scaffolds. We considered scaffolds are folded into DNA origami showing bright areas upper bands of scaffolds, therefore we chose 14, 16 and 18 mM MgCl2 for folding reactions of the mixture of scaffolds, staples, and T7-promoter GFP plasmids.
DNA folding reaction
Test whether DNA origami folded well --agarose gel electrophoresis
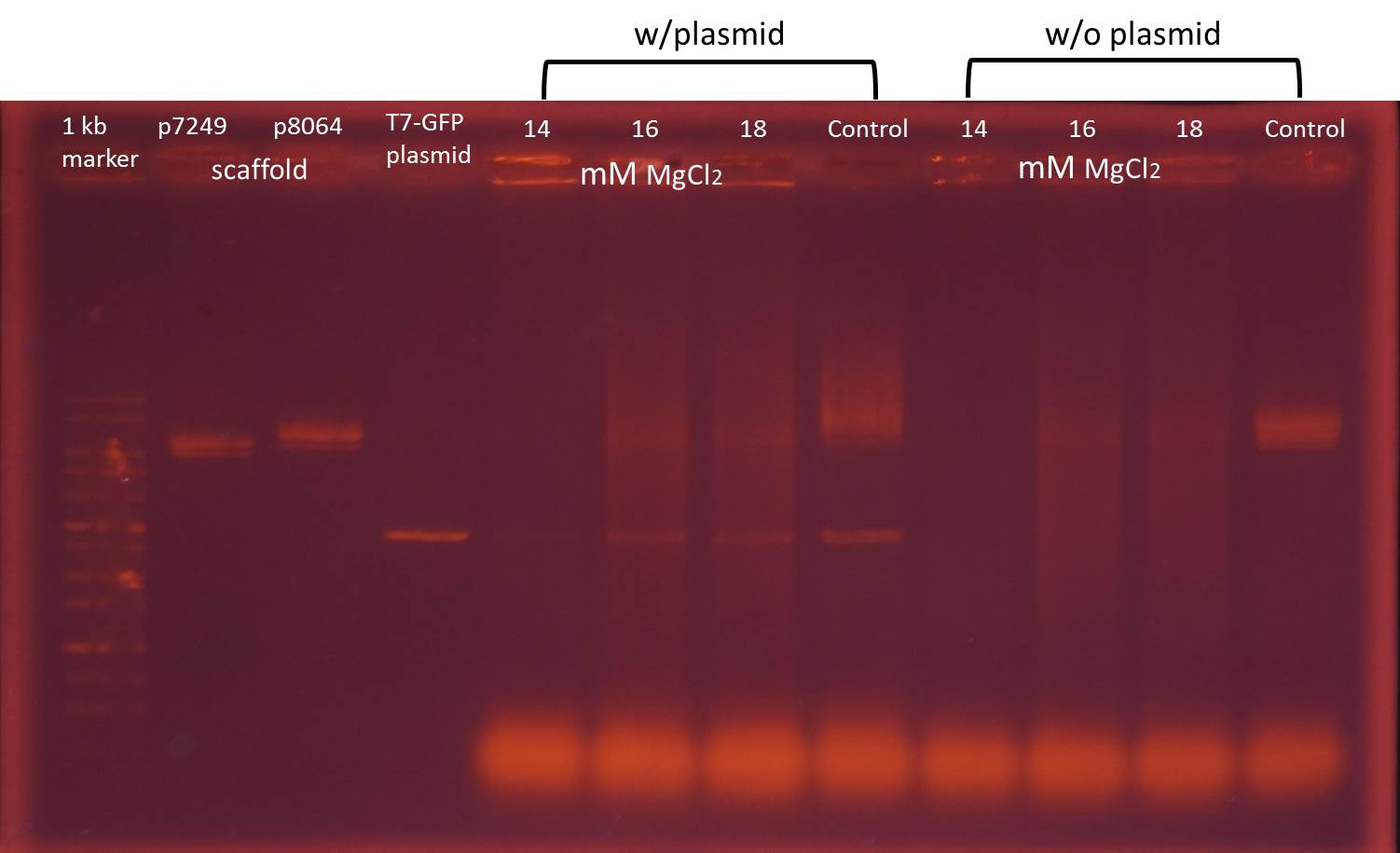
*control: the non-folding mixture of scaffolds, staples with or without T7-promoter GFP plasmids at 8 mM MgCl2. We use the control to test the effect of folding reaction.
The electrophoresis result of mixture with plasmids reveals that after the folding reaction, all bands of mixture are less obvious than only scaffolds and plasmids. In addition, there are bright areas upper bands of scaffolds in mixture at 16 and 18 mM MgCl2. Therefore we consider 16 mM MgCl2 is the best concentration for the folding reaction and choose 16 mM MgCl2 for further folding reactions in the functional test.
Functional Test
Test whether NanoNeedle successfully binds to cell wall of E. coli, penetrates its membrane and delivers T7-promoter plasmid into it.
We incubate E. coli with 7 combinations of origami components to test the function of NanoNeedle.
- T7-promoter GFP plasmid transformed E. coli is positive control
- without origami component (only folding buffer and MgCl2)
- with T7-promoter GFP plasmid only
- with non-T7-promoter GFP plasmid origami
- with non-folding origami components
- with origami in LB broth
- with origami in LB broth (Cp+)
(T7-promoter GFP plasmid carries chloramphenicol-resistant gene. Only E. coli acquiring plasmid from NanoNeedle can live in LB broth containing chloramphenicol)
Flow
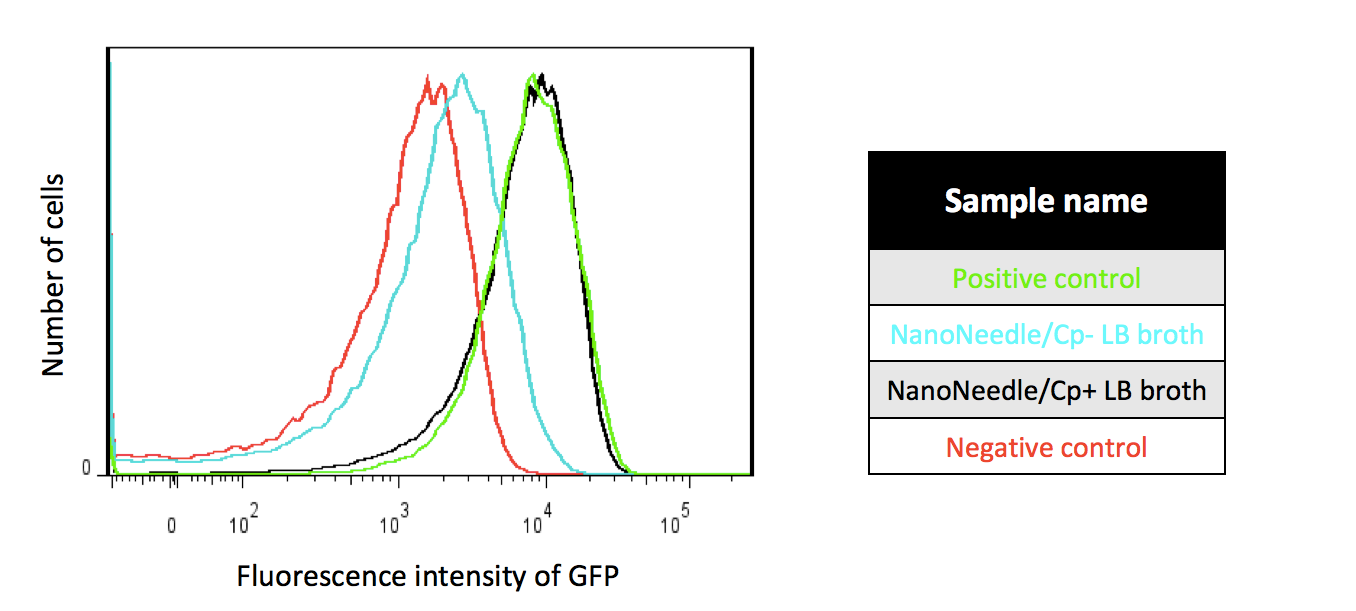
Positive control : T7-promoter plasmid contains GFP and anti-Cp genes. We transferred the plasmid containing T7-promoter GFP and anti-Cp genes into E. coli by transformation.
NanoNeedle/CP+ LB broth : After we incubated NanoNeedle with E. coli for 3.5 hours, we then incubated the former E. coli in LB broth containing Cp antibiotic.
NanoNeedle/CP- LB broth : After we incubated NanoNeedle with E. coli for 3.5 hours, we then incubated E. coli in LB broth without Cp antibiotic.
Negative control: We incubated E. coli only with 1X folding buffer, 16mM MgCl2 and dH2O.

Positive control : T7-promoter plasmid contains GFP and anti-Cp genes. We transferred the plasmid containing -promoter GFP and anti-Cp genes into E. coli by transformation.
Plasmid only: We incubated E. coli only with the plasmid containing T7-promoter GFP and anti-Cp genes.
NanoNeedle without the plasmid : We incubated E. coli with NanoNeedle without containing the plasmid consisting of T7-promoter GFP and anti-Cp genes.
NanoNeedle without folding reaction:We incubated E. coli with unfolded scaffolds, staples and the plasmid containing T7-promoter GFP and anti-Cp genes.
In order to understand whether NanoNeedle can deliver the plasmid into the bacteria, and proving that origami, folding reaction and the plasmid are essential elements for our project.We used various combinations to proceed functional test by flow cytometers and displaying our final result with histogram. Using the y-axis of the histogram to represent the number of the cells under the amount of fluorescence intensity, the x-axis represents the fluorescence intensity of GFP; choosing 509nm emission wavelength and the 488nnm excitation wavelength to carry out the experiment. For the plasmid construction, we used the plasmid containing T7-promoter GFP and anti-Cp genes (We call it “the plasmid” after). Since the plasmid contains anti-CP gene, when it successfully expresses the gene in the bacteria, they not only have resistance to Cp antibiotic but also expresses GFP gene.
As Figure 1 shows, under Cp-selection, E. coli injected into the plasmid are able to survive. Furthermore, after the survived bacteria proliferate, they not only have resistance to Cp antibiotic but also emit GFP signals as strong as positive control. This proves that NanoNeedle can deliver linear plasmid into bacteria and successfully express.
In addition, about 23.4% of E. coli express GFP which uses the negative control as threshold in the group with no Cp antibiotic added, implying that the success rate is merely 23.4%. The reasons might be the concentration of reactants or the folding success rate too low.Figure 2 indicates that the operation of Nanoneedle achieves our expectation. The fluorescence intensities of the following three sets, “NanoNeedle without containing the plasmid”, “NanoNeedle without folding reaction”, “
Plasmid only”, resemble the negative control. This indicates that only when we transfer plasmid into E. coli by correct folding process can we make the plasmid express.MTT assay
We used the MTT assay, the method of evaluating cell viability by a colorimetric assay, to test whether NanoNeedle would be toxic to human cells.
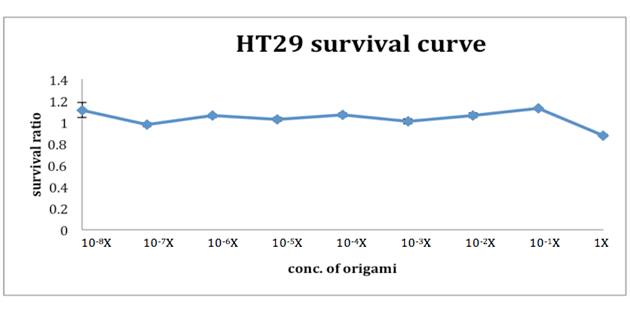
After incubating NanoNeedle with HT29 cells for 48 hours, we added 0.08N HCl and isopropanol and mixed thoroughly. The remaining living cells would appear purple. We used ELISA reader
to interpret the cell viability, also to acknowledge the survival rate of HT29 cells under different NanoNeedle solution. As the graph suggests, under 1X NanoNeedle solution there is a slight influence on HT29 cells, which the accurate survival rate is 0.876, but this did no harm to the cells. Below 0.1X NanoNeedle solution, they all didn’t do damage to HT29 cells. Surprisingly, they increased the cells growth instead. The result of the MTT assay lives up to our expectation. For Nanoneedle not only does no harm to human colon cells but works in human large intestine.TEM

According to the figure above, the stained substance is about 123 nm in length and 25 nm in width under Transmission Electron Microscope( TEM ). In comparison with the scale we expected, this consequence gives us much faith to surmise that what we see in the micro view might be our own Nanoneedle.